- The oxford vaccine trial is set to resume after being cleared by the UK Health department
- The trials were stopped earlier this month when a participant developed spinal cord problems
- The company has refused to give details of the participant but has assured that an independent investigation is being done.
- The investigation is done to understand whether the illness developed due to the vaccine or other issues
- The participant hasn’t disclosed whether she had this condition before.
- Meanwhile, scientists have reiterated that this type of side effect is common against viral vaccines.
- This shows that vaccine makers aren’t compromising of vaccine safety.
According to a BBC report, trials of a Covid-19 vaccine being developed by AstraZeneca and Oxford University will resume after being paused due to a reported side effect in a patient in the UK.
The Chain of Announcements
- On Tuesday, AstraZeneca said the studies were being paused while it investigated whether the adverse reaction was linked with the vaccine.
- But on Saturday, the university said it had been deemed safe to continue.
- Health Secretary Matt Hancock welcomed the news that the trials would resume.
Good news for everyone the Oxford vaccine trials are back up and running. This pause shows we will always put safety first. We will back our scientists to deliver an effective vaccine as soon as safely possiblehttps://t.co/RUtTE3sPim
— Matt Hancock (@MattHancock) September 12, 2020
“This pause shows we will always put safety first. We will back our scientists to deliver an effective vaccine as soon as safely possible,” he added.
The university said in a statement that it was “expected” that “some participants will become unwell” in large trials such as this one.
It added that the studies could now resume following the recommendations of an independent safety review committee and the UK regulator, the Medicines and Healthcare Products Regulatory Agency.
The World Health Organization (WHO) says nearly 180 vaccine candidates are being tested around the world but none has yet completed clinical trials.
Hopes have been high that the vaccine might be one of the first to come on the market, following successful phase 1 and 2 testing.
Its move to Phase 3 testing in recent weeks has involved some 30,000 participants in the US as well as in the UK, Brazil and South Africa. Phase 3 trials in vaccines often involve thousands of participants and can last several years.
Patient Suffering from Spinal Cord Inflammation
It would not disclose information about the patient’s illness for confidentiality reasons, but the New York Times reported that a volunteer in the UK trial had been diagnosed with transverse myelitis, an inflammatory syndrome that affects the spinal cord and can be caused by viral infections.
AstraZeneca Chief Executive Officer Pascal Soriot said on Thursday that the vaccine could still be available by the end of the year, reports Bloomberg
An independent safety board was reviewing whether the participant’s illness had been caused by the vaccine or was unrelated, he said.
Soriot said it wasn’t clear whether the participant had a condition called transverse myelitis, a suspected diagnosis. NIH Director Francis Collins told a Senate committee Wednesday the trial had been halted due to a “spinal cord problem.”
“We cannot disclose medical information about the illness for reasons of participant confidentiality,” Oxford said. “We are committed to the safety of our participants and the highest standards of conduct in our studies and will continue to monitor safety closely.”
Oxford said some 18,000 people have received “study vaccines” as part of the trials. It had begun a large phase 3 trial in the U.S. at the end of August, with the aim of enrolling 30,000 people.
AstraZeneca is one of several companies taking part in the U.S. government’s Operation Warp Speed program to fast-track a coronavirus vaccine. In May, the company inked a $1.2 billion deal with the U.S. to support clinical studies and supply 300 million doses of the vaccine. It has pledged to provide the vaccine on a not-for-profit basis during the pandemic and has lined up deals around the world to supply almost 3 billion doses.
New Rules To Stem Rising Infection
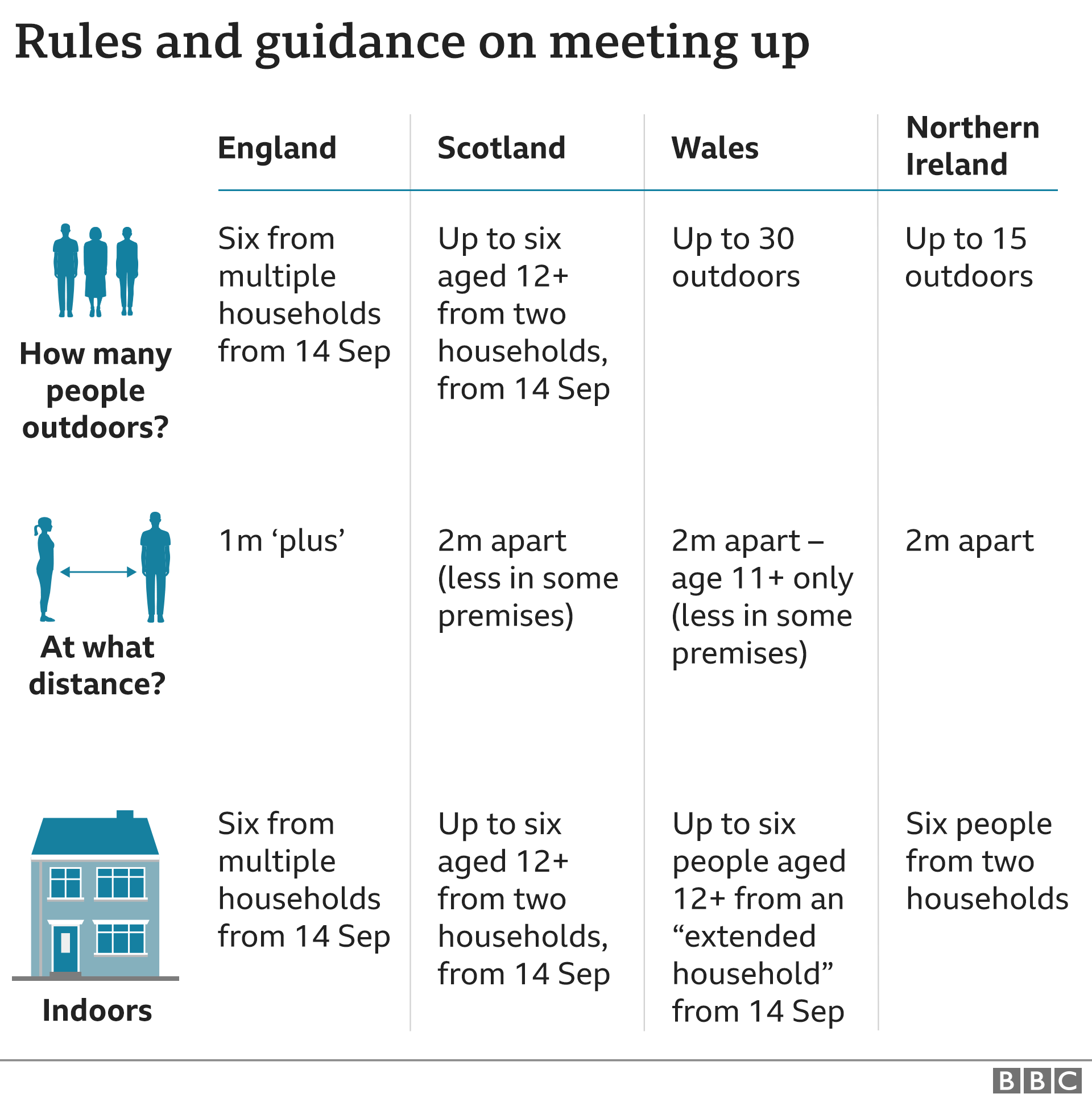
New “rule of six” restrictions intended to halt the rises are due come into force on Monday.
- In England indoor and outdoor gatherings of more than six people will be banned, except in certain circumstances such as for work or school. Those breaking the rules could be fined.
- In Scotland, socialising will be limited to a maximum of six people inside and outside – but unlike England they must be from two households, and children under 12 are exempt.
- In Wales, also from Monday, it will be illegal for more than six people from an extended household to meet indoors – but up to 30 can still meet outdoors.
- Localised restrictions for parts of Northern Ireland, including Belfast and Ballymena, are to come into force on Monday, aimed at reducing contacts between people in homes in the affected areas.
Cabinet Office minister Michael Gove agreed fines might be necessary to ensure people self-isolate when required.
He told BBC Radio 4’s Today programme: “I don’t want to see fines being levied, but even more I do not want to see people behaving in a way that puts the most vulnerable at risk.”
Did you subscribe to our daily newsletter?
It’s Free! Click here to Subscribe!

















