- Moderna mRNA vaccine shows promising results to be scaled up for phase 2 trials
- the trials in 45 people tested so far produced neutralizing antibodies well above the average antibody levels in normal covid patients
- Adverse effects like fever,chills and headaches were noticed in many but none of them were that serious
- Dr.Fauci says that this could be the winner vaccine we were looking for as it ticks the right boxes.
- Meanwhile, Moderna CEO has asked governments to come up with a distribution strategy as supply will be short
In a major development, Moderna Inc’s (MRNA.O) announced on Tuesday that US researchers reported their experimental vaccine for COVID-19 to be safe and effective in provoking immune responses in all 45 healthy volunteers in an ongoing early-stage study, reports Reuters
Outcome of the Study
According to a Bloomberg report, Moderna’s vaccine elicited antibodies in all people tested in an initial safety trial, federal researchers said Tuesday. Early results from tests of a vaccine Oxford is developing with AstraZeneca Plc will be published as soon as Thursday
While ITV.com referred to the Oxford results as “promising,” without providing any data, AstraZeneca shares climbed as much as 4.2% on the report. Other companies researching vaccines for the virus — including Pfizer Inc. and Merck & Co. — also rose, and optimism over Moderna lifted global equity markets.
- Volunteers who got two doses of the vaccine had high levels of virus-killing antibodies that exceeded the average levels seen in people who had recovered from COVID-19, the team reported in the New England Journal of Medicine.
- No study volunteers experienced a serious side effect, but more than half reported mild or moderate reactions such as fatigue, headache, chills, muscle aches or pain at the injection site.
- These were more likely to occur after the second dose and in people who got the highest dose.
Experts say a vaccine is needed to put an end to the coronavirus pandemic that has sickened millions and caused nearly 575,000 deaths worldwide.
Moderna was the first to start human testing of a vaccine for the novel coronavirus on March 16, 66 days after the genetic sequence of the virus was released.
A Winner Vaccine
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, whose researchers developed Moderna’s vaccine candidate, called the results “good news,” noting that the study found no serious adverse events and the vaccine produced “reasonably high” levels of virus-killing or neutralizing antibodies.
Read More: US Will Meet COVID19 Vaccine Goal of 300 Million Doses
“If your vaccine can induce a response comparable with natural infection, that’s a winner,” Fauci said in a telephone interview. “That’s why we’re very pleased by the results.”
Moderna shares jumped more than 15% in after-hours trading on Tuesday.
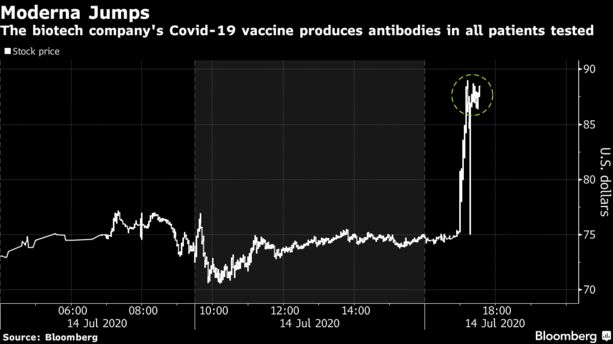
CEO urges govt to act on the allocation
Moderna Inc’s (MRNA.O) chief executive said on Wednesday that he expects governments will make the decision on how to allocate COVID-19 vaccines while supplies are scarce.
Chief Executive Stéphane Bancel said on a conference call to discuss early stage clinical results for his company’s experimental vaccine that Moderna could potentially work with the Centers for Disease Control and Prevention or the Department of Defense to distribute it in the U.S.
“This is not for a private company to make those decisions. We will not have the right data to be able to make those important decisions,” he said.
Government Support for Large Scale Trials
The U.S. government is supporting Moderna’s vaccine with nearly half a billion dollars and has chosen it as one of the first to enter large-scale human trials.
A successful vaccine could be a turning point for Cambridge, Massachusetts-based Moderna, which has never had a licensed product.
How does this vaccine work?
Moderna’s shot, mRNA-1273, uses ribonucleic acid (RNA) – a chemical messenger that contains instructions for making proteins. When injected into people, the vaccine instructs cells to make proteins that mimic the outer surface of the coronavirus, which the body recognizes as a foreign invader, and mounts an immune response against.
How did the present vaccine trials work?
- The results released Tuesday involved three doses of the vaccine, tested in groups of 15 volunteers aged 18-55 who got two shots, 28 days apart.
- The groups tested 25, 100 or 250 micrograms of the vaccine.
- Adverse events after the second dose occurred in seven of the 13 volunteers who got the 25-microgram dose, all 15 participants who received the 100 microgram dose and all 14 who got the 250 microgram dose.
- In the highest-dose group, three patients had severe reactions such as fever, chills, headache or nausea.
- One of these had a fever of 103.28 Fahrenheit (39.6 C).
“We didn’t see any events that are characterized as serious adverse events,” said lead author Dr Lisa Jackson of Kaiser Permanente Washington Health Research Institute in Seattle, referring to reactions that require hospitalization or result in death.
Limitations of the Study
One significant limitation of the data is it includes information only from the first 45 patients in the study, all of whom were from age 18 to 55.
Results from a second portion of the phase 1 trial that included older people — a key demographic for any Covid-19 vaccine, given the high death rate in older patients — are not available yet.
William Haseltine, a former Harvard Medical School researcher who chairs Access Health International, said the levels of neutralizing antibodies produced were “respectable” and possibly protective. But he said “the jury is out” on the vaccine’s safety.
“Man, that is a lot of adverse events,” said Tony Moody, a doctor and researcher at the Duke Human Vaccine Institute. He said it would be “unusual” for a vaccine to have this rate of side effects.
Encouraging Levels of Antibodies Produced
On the plus side, Moody said that the antibody levels produced were “really encouraging.” The neutralizing antibody levels in the trial produced were equivalent to the upper half of what’s seen in patients who get infected with the virus and recover, according to the results published Tuesday in the New England Journal of Medicine.
Moderna’s stock has almost quadrupled in value this year on hopes that the company’s vaccine will gain rapid approval. The vaccine will move into a much larger late-stage trial later this month that’s likely to determine whether it’s fit for commercial use.
Although stimulating production of neutralizing antibodies doesn’t prove a vaccine will be effective, it’s considered an important early step in testing. The side effects reported weren’t severe enough in the majority of patients to preclude further testing, according to the report by researchers from the NIAID.
Go Ahead To Phase 2 Trials?
In June, Moderna said it selected the 100-microgram dose for its late-stage study to minimize adverse reactions.
At that dose, Moderna said the company is on track to deliver about 500 million doses per year, and possibly up to 1 billion doses per year, starting in 2021, from the company’s internal U.S. manufacturing site and strategic collaboration with Swiss drugmaker Lonza (LONN.S).
“It’s a good first step,” said Dr William Schaffner, a vaccine expert at Vanderbilt University Medical Center who was not involved in the study.
“There’s nothing here that would inhibit one from going ahead to the Phase 2/Phase 3 trials,” he said.
- In April, Moderna expanded the Phase 1 trial to include adults over 55, who are more at risk of serious disease, with the aim of enrolling 120 volunteers.
- Moderna said it will follow study volunteers for a year to look for side effects and check how long immunity lasts.
- Moderna started its phase 2 trial in May and expects to start a phase 3 trial on July 27.
Phase 1 trials aim to ensure a treatment is safe and help determine an effective dose. Phase 2 trials test a treatment in a larger group and get an early read on effectiveness. Phase 3 trials are conducted in a large group of individuals to confirm efficacy and identify rare side effects.
Moderna’s Phase 3 trial will be conducted in 30,000 volunteers.
Did you subscribe to our daily newsletter?
It’s Free! Click here to Subscribe!




















